When parts are just machined, the surface is usually rough (we call it "machined state"). If you want them to be beautiful, perform well and be durable, you have to rely onsurface finishing. Simply put, this is the process of upgrading the surface of parts.
What I study every day is the doorway behind these processes:
- They either remove a little material by physical or chemical methods,
- or add a layer of new substances,
- or directly change the atomic structure or composition of the surface of the material.
The purpose is clear:To improve the appearance texture, and more importantly, to enhance key performance, such as rust prevention, wear resistance, hardness improvement, conductivity improvement, and even friction reduction.
Why is the anodized film hard and difficult to peel off, while the spray paint is easily scratched? Why can plastic parts have a metallic luster afterelectroplating? The root of these differences lies in the fact that their working principles at the atomic or molecular level are completely different. Understanding these principles is like having an "X-sight" that can see through the essence of each process. Only by understanding the principles can you choose the most appropriate solution for your product at the most appropriate time.
Summary of Key Points:
| Working principle classification | Typical process | Fundamental changes |
| Mechanical force reshaping | Sandblasting, polishing, and drawing. | Physically removing or reshaping surface materials to alter the microstructure. |
| Electrochemical reaction | Anodizing, electroplating, electrolytic polishing. | Using electric current to drive chemical reactions and generate or remove a layer of material on the surface. |
| Chemical conversion | Passivation, blackening, chemical etching. | By chemical reactions, the surface material is transformed into a new layer of substance. |
| Material attachment | Spray painting, powder coating, PVD coating. | Cover the surface of the part with external materials to form an independent coating. |
This article will answer your questions:
- This guide will explain in detail how surface finishing works.
- I will explain the four core principles of surface finishing to you in words that everyone can understand.
- Let's share a real case to see how we use a combination of different principles to solve the same difficult problem. This idea may make you look at the problem from a different angle.
- Finally, the principle of surface finishing process that everyone often asks is also answered.
Why Trust This Guide? Because JS Understands The Principle and The Actual Combat
At JS, I come into contact withsurface finishing equipmentevery day. But for me, it is not as simple as turning on the machine and pressing buttons.
What I really think about is: Why do we set it up like this?
- For example, how will adjusting the current of anodizing affect the tiny holes in the oxide film?
- What are the different effects of sand blasting with different hardness on the residual stress on the surface of the part?
- These understandings at the principle level allow me to provide not only equipment operation technology, but also solutions to solve the problem from the root.
This guide contains the essence of my experience solving customer problems. Have customers encounteredPVD coatingsthat don't stick well and fall off at the touch? I've studied it thoroughly. The plating solution can't flow into the small corners of complex parts, and the thickness of the coating is uneven? I've also tackled this hard bone.
It is these experiences that have delved into the depths of the problem that have transformed me from an equipment operator to a technology optimizer. What you read is the core knowledge that I have repeatedly verified with experience and experiments.
"A core concept of Amy Smith, a professor of mechanical engineering at MIT: real engineering is not about remembering solutions, but about understanding the principles behind them and creating new solutions."
This guide is to help you understand the essence of metal surface finishing techniques and make smarter choices.
Principle 1: Mechanical Action
It's like we use hand tools tocarve and polish the surface of a partbit by bit, and directly use force to change it. This is the most basic and intuitive method in our surface finishing engineering.
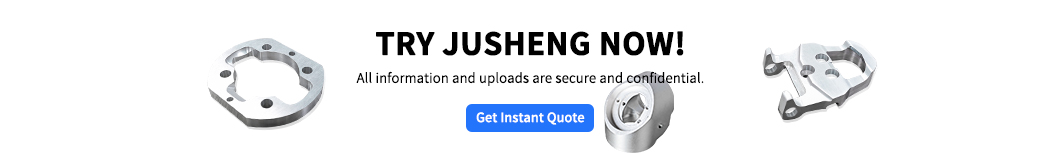
How does sandblasting work? —Billions of micro-impacts
Imagine this: We use high-pressure gas to blast out a handful of tiny particles (such as glass beads or aluminum oxide sand). These small particles are like countless tiny hammers, hitting the surface of the part at high speed. Each impact leaves a tiny dent. After thousands of hits, these small pits are connected together, and the originally bright orflawed surfacebecomes a uniform matte or frosted surface.
What are the big benefits?
In addition to changing appearance, more importantly, this impact primarily compresses the material at the surface of the part, causing compressive stress in this layer of material. This pressure can greatly increase the part's resistance to fatigue and cracking, allowing the part to last longer.
Image understanding:Think of using extremely fine sand to impact the surface uniformly, removing gloss and flaws, leaving a uniform texture.
How is polishing accomplished? —From "peak cutting" to "valley filling"
Working principle:
- It will be more precise. We use a polishing wheel, with polishing wax or abrasive paste, to rub the surface of the part at high speed. The small "peaks" (protrusions) on the surface are gradually worn away, and the surface becomes flatter and smoother, and finally reflects like a mirror.
- There is a more powerful technique, e.g,electrolytic polishing. It relies on electricity and chemical solutions to dissolve the surface bumps, producing a surface that is extremely even and smooth, and is particularly adept at dealing with complex shapes.
Core function:The purpose is fairly evident, e.g, to achieve extreme smoothness and flatness, reduce friction, improve appearance, or create a good basis for subsequent processing (for instance, electroplating, spraying).
For example: It is like being a "bulldozer" in the microscopic world, flattening the wavy and rough "terrain" into a smooth "plain.".
Sandblasting/shot peening achieves surface modification and strengthening through impact, while polishing/grinding is committed to extreme smoothness. Mastering the principles of these mechanical actions is the key to surface treatment engineering.
"Choose the right method to solve practical problems, don't just look at the surface results, but also understand the reasons behind them. Want to talk in depth about how to better treat your parts? Feel free to contact our JS engineers!"
Principle 2: Electrochemical Reaction
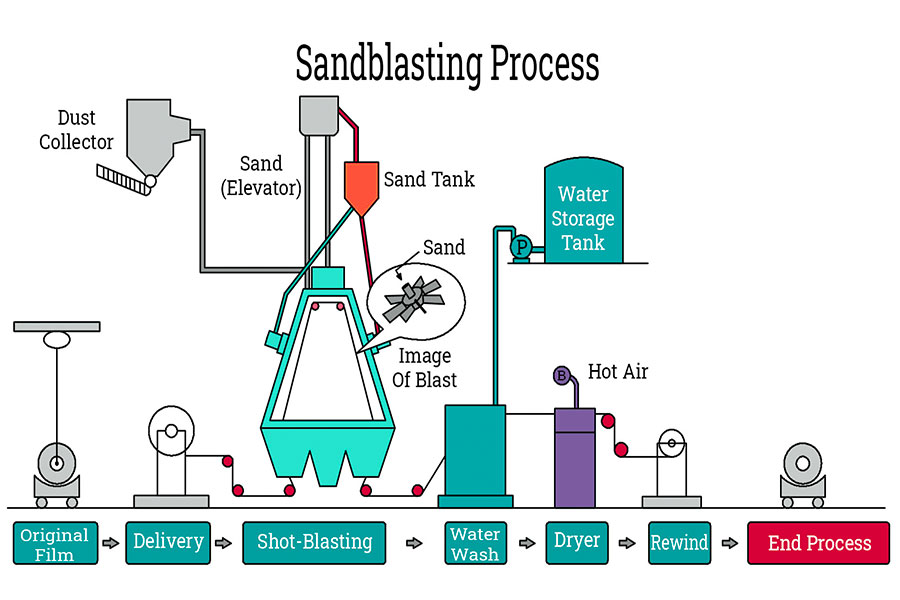
Today, let's talk aboutthe most magical principle in surface treatment - electrochemical reaction. This is not magic, but our core technology of using electric current to drive atomic movement and grow a new layer on the surface of parts.
Anodizing: Building a protective layer on the surface of aluminum
Process:
- Connect the aluminum parts to the positive pole (anode) of the power supply, soak them in a specific acid solution (electrolyte) and turn on the power. Magical things happen: the current will drive the aluminum surface to combine with the oxygen in the solution, and directly grow a dense and hard aluminum oxide (Al₂O₃) layer on thealuminum substrate.
- This film is converted from aluminum itself, so it has a super strong bonding force with the substrate. And this film is naturally born with countless nano-scale pores, which is just convenient for us to dye it with various colors later.
Core value:This layer of "native armor" greatly improves the corrosion resistance, wear resistance and insulation of aluminum, and can also be dyed and beautified. It is one of the most common and effective means to protect aluminum parts.
Analogy: It is like using electrical stimulation to allow aluminum to "grow" a layer of ceramic protective shell from the surface itself, rather than simply covering it.
Electroplating: Metal coating of parts
Process:
- Connect the parts that need to be plated to the negative pole (cathode) of the power supply, and connect the metal to be plated to the positive pole (anode), and immerse them together in a solution containing this metal ion. Once the power is turned on, the metal block of the anode will dissolve and become ions to enter the solution.
- At the same time, the metal ions in the solution are attracted to the surface of the cathode (parts), and after obtaining electrons, they turn back intometal atoms, and "accumulate" on the surface of the parts layer by layer and very evenly.
Core value:
Electroplating can give parts new surface characteristics: chrome plating enhances wear resistance and glossiness, nickel plating improves corrosion resistance, and gold/silver plating is used for conductivity and decoration. It can accurately control the thickness and uniformity of the coating,and is one of the most basic and flexible processes in the field of plating & surface finishing.
Take a look at the uniformity levels that can be achieved by common coatings:
| Coating type | Typical thickness range (μm) | Thickness uniformity (ratio of high/low current density areas) | Main functional attributes |
| Decorative chromium | 0.25 - 1.0 | 3:1 - 5:1 | High gloss, wear-resistant, decorative. |
| Hard Chrome | 5 - 500+ | 1.5:1 - 3:1 | Extremely high hardness (800-1000 HV), wear-resistant. |
| Bright nickel | 5 - 25 | 1.2:1 - 2:1 | Good corrosion resistance and mirror gloss. |
| Acid copper plating | 5 - 50+ | 1.1:1 - 1.5:1 | Excellent coverage, flatness, and conductivity. |
Data source: American Society for Metals - ASM Handbook, Vol 5 (Note: The closer the thickness ratio is to 1:1, the more uniform the distribution of the coating in different areas of the part.)
Image metaphor: This is similar to providing metal ions "immigrate" from the anode (dissolve), move through the electrolyte, and exactly "settle" (deposit) on the "new world" of the cathode pieces at the behest of the electric field.
"Anodic oxidation forms a protective ceramic layer via in-situ reaction and electroplating achieves accurate and controllable metal ion deposition. Underlying the nature of these electrochemical reactions is the root answer to sophisticated surface finishing engineering. If you need aluminum protection or request for specified metal coating performance, please contact JS, we will apply the power of electrochemistry to design the most appropriate solution for you!"
Principles 3 & 4: Chemical Conversion & Material Addition
Regarding the two principal methods of surface protection,chemical conversion (internal work) and adding material (external force), I am about to explain two such processes, which I use on a regular basis:
Passivation(chemical conversion): stimulating the "self-healing" property of stainless steel
- This is not plating something onto the outside. What I actually do is submerge the stainless steel components in a specific acid solution (such as nitric acid or citric acid solution).
- This acid bath will dissolve only the more "active" metal components such as iron on the surface of the parts, leaving the more chromium components on the surface.
- These enriched chromium, upon contact with oxygen in the air, will naturally form a very thin, very dense, and very stable chromium oxide protective layer (Cr₂O₃), not even visible to the naked eye.
"Professor Herbert H. Uhlig emphasized in his classic book Corrosion and Corrosion Control: The excellent corrosion resistance of stainless steel fundamentally depends on this thin, tough, self-healing passivation film."
The secret of this film is that when it is scratched lightly, it can use the oxygen in the air to "self-heal" and re-form a protective layer. It is stainless steel's "magic trick."
Spraying/powdering (material adhesion): Put on a customized "protective coat" for parts
The core of these two methods is to add a physical protective layer on the outside of the parts.
(1) Spraying:
It is to spray the liquid paint into a fine mist and evenly cover the surface of the part. After the solvent inside evaporates, or the paint itself undergoes a chemical reaction, it hardens and solidifies into a paint film.
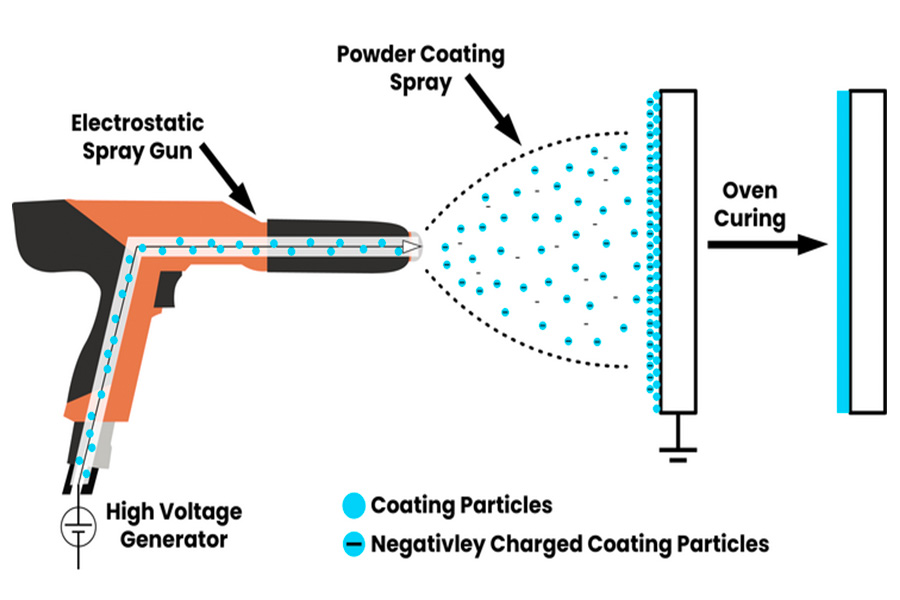
(2) Powdering (electrostatic spraying):
- This is more commonly used and interesting. I charge the dry plastic powder with static electricity and then spray it on the grounded part. The electrostatic attraction will make the powder firmly adhere to the surface of the part.
- Then send the part into the baking oven, the powder is melted and leveled by heat, and finally cooled and solidified, forming a very uniform andstrong coating, like wearing a tight protective suit for the part.
"Passivation stimulates stainless steel to form a protective film that can repair itself. Painting/powder spraying adds an extra layer of solid outer coat to isolate the environment. At JS, we accurately select the most suitable protection solution based on the material of the parts and the use environment to ensure the service life of the product. Want to know which one is best for your needs? Feel free to talk to us!"
Actual Combat Case Analysis: The Anti-Corrosion Challenge of an Underwater Detector Shell
I would like to share with you the true case we just resolved, talking aboutunderwater detector shell anti-corrosion issues and solutions that we learned. This issue clearly made me realize how important process principles impact can be on failure or success.
Customer challenge: Maintain structural integrity in seawater and salt spray for a long time
We have a client who has come up with an underwater detector shell made of analuminum alloy (6061). The design is good but must last for a long time in high-salt seawater. The client is worried that:
- Long-term corrosion resistance: The shell must withstand seawater erosion and salt spray so that it will possess long-term structural integrity and failure.
- Clear logo: The logo and text on the shell must not be obscured by the protection process and should be viewable clearly at all times.
- This corrosion in seawater is no laughing matter, especially when the aluminum alloys are involved. When the wrong protection process is used, the lifespan of the product will be shortened considerably.
Solution evaluation: The key lies in the formation principle of the protective layer
At that time, the customer mainly considered two common methods. I carefully analyzed their working principles and potential risks:
Solution A: Spray high-performance marine epoxy paint
- Principle: Physical isolation. Rely on the coating itself to isolate seawater from the substrate.
- Risk point: This protective layer is very fragile. Even if a small hole as big as a pinhole is scratched, seawater can get in and electrochemical reaction (galvanic corrosion) will occur. The result is that the paint starts to fall off under the paint film, and eventually a large area fails.
Solution B: Ordinary anodizing
- Principle:Electrochemical conversion. A layer of aluminum oxide protective film is generated on the aluminum surface, and this film is very well combined with the aluminum itself.
- Risk point: The problem is that the film grown by ordinary anodizing has limited thickness and tightness. In seawater with high chloride ion concentration, this film is still easy to be "punched through", forming small corrosion points, which may eventually lead to failure.
JS's optimal solution: hard anodizing + sealing
Based on a deep understanding of how surface treatment really works, we did not choose the above two, but recommended and implemented an upgraded electrochemical solution: hard anodizing + sealing.
Why is it the optimal solution?
(1) Hard anodizing:
- This process is very "hardcore": it operates under special conditions of low temperature and high current density.
- The effect obtained: a super thick, super dense, and extremely small pore oxide layer is generated on the surface of the aluminum alloy. The defense of this "armor" itself is several levels stronger than that ofordinary anodizing.
(2) Sealing:
- Although the pores of the hard oxide film are small, there are still tiny channels under the microscope.
- The key step: We treat this oxide film with hot water or a specific chemical solution. This step will cause the aluminum oxide in the micropores on the surface of the oxide film to absorb water and expand, completely blocking and sealing those nano-scale tiny pores. Seawater has no chance to penetrate.
Why is our method better?
- Spray painting (Scheme A) is a physical barrier. Once it is damaged, the aluminum substrate inside will suffer, and then the whole will fail.
- Our hard anodizing + sealing allows the aluminum to grow a complete, dense, and non-through-pore ceramic surface layer. Even if the surface is accidentally scratched, the damage is usually limited to the scratch, will not spread to the surrounding area, and will not peel off as a whole.
The protection ability is very different!As a professionalsurface finishing tools, we are well aware that this step plays a decisive role in eliminating pores and achieving long-term protection.
Final result: durability far exceeds expectations!
The comparison of test data after the implementation of the plan clearly shows its advantages:
| Evaluation indicators | Plan A (High Performance Epoxy Paint) | JS scheme (hard oxidation+sealing) | Core advantages |
| Salt spray resistance test | Bubbles appear after 500 hours. | >3000 hours without any signs of corrosion. | Increase lifespan by more than 6 times. |
| Membrane substrate adhesion force | 3B level (with peeling on the grid). | 5B level (highest, no peeling). | Strong resistance to mechanical damage. |
| Logo clarity | Spraying may mask details. | Laser engraving before oxidation, perfectly preserving details. | Balancing functionality and aesthetics. |
"This case proves once again that a deep understanding of the working principle of surface treatment technology is the key to cope with the challenges of extreme working conditions. When your product faces a harsh corrosive environment,JS Companyrelies on solid process principle analysis to provide you with proven protection solutions. Welcome to discuss your challenges with us."
FAQ - Answer Your Process Principle Questions
Why is the electroplating layer thicker at sharp corners?
When I do plating and surface finishing, I often see that the coating is particularly thick at the sharp corners and protrusions of the parts. This is mainly because the current is unevenly distributed on the surface of the part, and it will naturally concentrate on those sharp and protruding places (this is called the tip effect), resulting in a particularly high current density there. When the current is strong, more metal ions are attracted to deposit, and the coating naturally becomes thicker.
Therefore, when designing electroplated parts that require precise matching, we must make rounded arcs in advance at those sharp corners (that is, add R angles), which can effectively disperse the current and make the coating thickness of the entire surface more uniform.
The anodized film itself is insulating, so why can it be dyed?
This is a very good question!
- Although the aluminum oxide film generated by anodizing is insulating in itself, nanoscale pores are actually generated inside this film during its formation process, which extend from the surface to the place close to the metal substrate. It is these tiny holes that allow dye molecules to penetrate and adsorb on the inner wall of the pores.
- After dyeing is completed, we still need to carry out a key sealing step to seal the openings of these small holes. In this way, the dye is permanently locked in the originally transparent oxide film.
- So the color you see is actually the effect of light passing through this transparent ceramic shell and irradiating the sealed dye inside. This is the key to why the insulating oxide film can also be dyed with rich colors.
What is the essential difference between PVD coating and electroplating?
As an engineer at JS, let me talk about the fundamentaldifference between PVD coating and electroplating:
Although both add a layer of material to the surface of the workpiece, the principles and environment are very different.
Electroplating is carried out in a liquid tank, relying on electrochemical reactions to deposit metal ions.
And what about PVD?
- It is a dry physical process operated in a high vacuum environment: we directly use methods such as ion beams to bombard the solid target material (the material you want to plate) into atoms or molecules in a gaseous state, and then let them splash in the vacuum and deposit on the surface of the workpiece to form a thin film.
- Because PVD operates in this way, its film layer is usually denser, harder, more firmly bonded, and more environmentally friendly, but correspondingly, the equipment investment and cost are much higher. So which one to choose depends on specific needs and cost considerations, and there is no absolute good or bad.
Summary
In the final analysis, being familiar with varioussurface finishing processesis basic skills, but only those who really understand the working principles behind each technology can be considered experts. Different working principles directly determine the performance ceiling and where it is most suitable for use. Only by mastering these can you be more confident when developing products and make more accurate and forward-looking choices.
Take action:
If the pain point is not only the appearance, but is stuck on performance issues: Don't hesitate, you need a partner who truly understands the principles of surface treatment engineering, not just selling equipment.
Talk to JS:As a professional surface treatment equipment company, our team of engineers is happy to share knowledge and experience.Please contact usimmediately to discuss the details of your project.Let us start from the most basic principles, help you sort out your needs, and design a truly reliable and effective manufacturing solution.
Disclaimer
The contents of this page are for informational purposes only.JS seriesThere are no representations or warranties, express or implied, as to the accuracy, completeness or validity of the information. It should not be inferred that a third-party supplier or manufacturer will provide performance parameters, geometric tolerances, specific design characteristics, material quality and type or workmanship through the Longsheng Network. It's the buyer's responsibilityRequire parts quotationIdentify specific requirements for these sections.Please contact us for more information.
JS Team
JS is an industry-leading companyFocus on custom manufacturing solutions. We have over 20 years of experience with over 5,000 customers, and we focus on high precisionCNC machining,Sheet metal manufacturing,3D printing,Injection molding,Metal stamping,and other one-stop manufacturing services.
Our factory is equipped with over 100 state-of-the-art 5-axis machining centers, ISO 9001:2015 certified. We provide fast, efficient and high-quality manufacturing solutions to customers in more than 150 countries around the world. Whether it is small volume production or large-scale customization, we can meet your needs with the fastest delivery within 24 hours. chooseJS TechnologyThis means selection efficiency, quality and professionalism.
To learn more, visit our website:jsrpm.com